Measuring Brain Activity Pattern May Help Identify More Effective Anti-epileptic Therapies
Incorporating brain activity pattern analysis in chemical compounds screenings for Dravet syndrome and other epilepsies can help predict therapies’ effectiveness and minimize chances for off-target effects.
That finding comes from the study “Brain activity patterns in high-throughput electrophysiology screen predict both drug efficacies and side effects,” which was published in the journal Nature Communications.
Developing drugs for brain diseases has been supported by rodent studies using the animals’ behavior as a readout. However, this can be a limiting strategy because it fails to detect underlying deficits and potential off-target effects. That means the therapy works on other pathways rather than those it was designed for.
The authors believe that direct high-content readouts of neural activity and brain activity patterns represent an attractive alternative to behavior-based screenings because they may capture more accurately disease pathology, drug activity, and side effects.
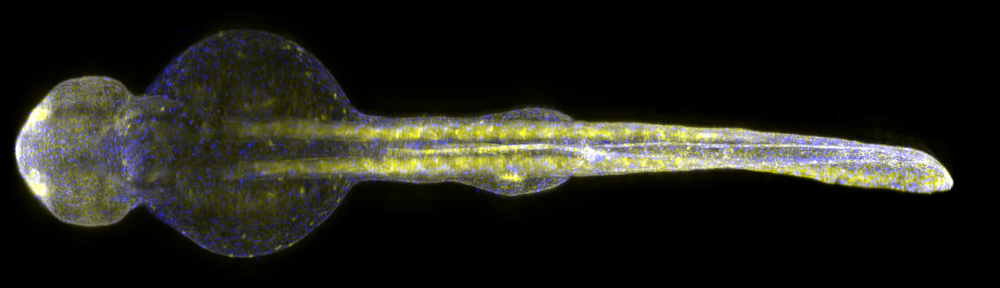
Performing this type of analysis in search for new drugs via high-throughput screening — where you can test thousands of potential compounds in parallel — is not feasible in rodent-based models, due to its high cost, low throughput, and need for high amounts of the compounds to test. As such, researchers used larvae of zebrafish, a small fish model organism that has emerged as an important new vertebrate model for CNS [central nervous system] diseases and drug screening. Zebrafish larvae are very small (only two millimeters long), allowing researchers to analyze several animals in parallel.
The team used the zebrafish model to determine if measuring their brain activity pattern analysis could be used for screening potential new drugs. Researchers developed a platform capable of monitoring brain activity simultaneously in many larvae for long periods of time using microelectrodes that are placed into the animals’ brains. They then developed computer programs to detect drugs’ effects on brain activity patterns, allowing them to monitor the in vivo consequences of neuroactive compounds on brain activity patterns in real-time and quantifying the effectiveness while detecting potential side effects.
The zebrafish larvae carried a mutation on the SCN1A gene that is linked to several forms of childhood epilepsies, including Dravet syndrome. The mutated larvae showed the same light sensitivity as reported for Dravet syndrome children. Photosensitive seizures, which can be triggered by flashing stimuli, bright light, or strong contrast between darkness and light, have been reported in 30 to 40 percent of patients with Dravet syndrome and often are associated with more severe outcomes.
“In our experiments on the larvae with the gene defect, we found the typical signals that arise during seizures. That was not the case in the healthy larvae,” study lead author Mehmet Fatih Yanik, PhD, Massachusetts Institute of Technology and ETH, Zurich, said in a press release.
Researchers found that the complexity of local brain activity in the mutant larvae was considerably less complex than in healthy larvae. The complexity within brain nerve cells network is a hallmark of a healthy brain and, as such, patients with Parkinson’s disease or schizophrenia also are characterized by less complex brain waves.
The authors hypothesized that compounds which increase complexity of brain signals may carry therapeutic potential, one that can be measured by brain signals directly if included as a therapeutic readout. Supporting this notion are the results they obtained after performing a screening for antiepileptic drugs. By analyzing brain activity patterns they identified 31 potential instances that influence brain waves, with some compounds increasing the complexity of brain activity.
The study’s results revealed a strong correlation between compounds that are effective based on brain activity patterns and those that significantly reduce seizure-associated behaviors with minimal side effects.
Moreover, one of the team’s top hits (fluoxetine; a selective serotonin reuptake inhibitor), recently has been reported to cause a marked reduction in seizures in an adult woman with Dravet syndrome.
Overall, this multiparametric screening approach points toward several promising new therapeutics for Dravet syndrome and other epilepsies, illustrating the power of using brain activity pattern analysis for central nervous system drug discovery.